Dealing with pool and hot tub water chemistry issues is sometimes confusing and occasionally frustrating because of the many critical components involved. A basic understanding of this topic is often not enough. Sometimes it requires ‘diving in’ a little deeper and gaining a better understanding of what it takes to maintain healthy, balanced water. The first key players in attaining this goal are sanitation and oxidation.
Sanitation and oxidation
Although many service technicians think ‘sanitation’ and ‘oxidation’ are one in the same, there is a distinct difference between the two. Sanitation is the complete destruction of bacteria, germs, viruses, algae, etc., that may enter the water. These nasty components can be potentially harmful to swimmers. Sanitizing them (i.e. killing them) removes this possibility. Oxidation is the process of oxidizing (i.e. burning off) organics that may get into the water such as hair, oils, lotions, sunblock, leaves, etc. By ridding the water of these contaminants, it eliminates the main source of eye, nose, and skin irritation. The good news is with proper sanitation and oxidation these problems do not exist.
Consider the following:
- the average person can introduce more than 50 billion bacteria into pool water;
- the average-sized dog is equal to 50 people—that means 250 billion bacteria enter the water when Fido decides to jump in for a swim.
Although there are many products available for sanitizing and oxidizing pool/hot tub water, there are actually only two chemicals that can perform both of these processes at the same time—chlorine and bromine.
First, here is a look at the six forms of chlorine currently available:
- Chlorine gas (Cl2)
- Sodium hypochlorite (NaOCl)
- Calcium hypochlorite [Ca(OCl)2]
- Lithium hypochlorite (LiOCl)
- Trichloroisocyanuric acid, a.k.a. Trichlor (C3N3O3Cl3)
- Sodium dichloroisocyanurate, a.k.a. Dichlor (NaC3N3O3Cl2)
All chlorine products react with water to produce the same acid—hypochlorous acid (HOCl):
H2O + Cl2 → HOCl + OCl–
HOCl is a very powerful sanitizer/oxidizer, whereas the OCl– (hypochlorite) portion has only one percent of HOCl’s ability to kill germs.
When considering which product to use for the pool and/or hot tub being serviced, it is important to know that each of these products has its own unique characteristics. One of the primary issues with chlorine is the difference in the available chlorine content (ACC) in the product and the effect it has on the water’s pH. (see Figure 1 below)
| Product | Available Chlorine Content (ACC) | pH |
| Chlorine | 100 % | |
| Sodium Hypochlorite | 10 to 12 % | 11 to 13 |
| Calcium Hypochlorite | 45 to 78 % | 8.5 to 11.8 |
| Lithium Hypochlorite | 35 % | 10.8 |
| Dichlor | 55 to 63 % | 6.7 |
| Trichlor | 90 % | 2.8 to 3.5 |
Figure 2 (below) shows how the water’s pH determines the proportions of HOCl and OCl–.
| pH | % HOCl | % OCl- |
| 7.0 | 79.3 | 20.7 |
| 7.2 | 70.7 | 29.3 |
| 7.5 | 54.8 | 45.2 |
| 7.8 | 37.8 | 62.2 |
| 8.0 | 27.7 | 72.3 |
Chlorine gas
Chlorine gas is normally a pale greenish-yellow and has a pungent odor, which can burn the eyes, nose, throat, and lungs if handled improperly. Its use in North America has diminished greatly in the past few decades.
When added to water, chlorine gas has the following chemical reaction:
Cl2 + H2O → HOCl + HCl
Advantages
- least expensive source for creating HOCl
Disadvantages
- high capital costs for application equipment;
- highly regulated (with associated costs);
- lowers pH and total alkalinity (TA) dramatically at application site; and
- meticulous safety procedures required (no known injuries from licensed applicators).
Sodium hypochlorite
Sodium hypochlorite, an inorganic chlorine, is stable only in solution, but loses strength over time, degrading to 10 to 12 percent strength at point of use. It is also often referred to incorrectly as ‘liquid chlorine.’
When added to water, sodium hypochlorite has the following chemical reaction:
NaOCl + H2O → HOCl + Na+ + OH–
Advantages
- completely soluble in water;
- after chlorine gas, it is the least costly source of HOCl; and
- leaves no residue in pool water.
Disadvantages
- bulky and heavy; ≈ 90 percent water and caustic;
- creates strong acid demand by raising pH due to use of sodium hydroxide to manufacture and stabilize the bleach; and
- decomposes quickly in storage.
Even though this is commonly referred to as ‘bleach,’ it must be noted that laundry-grade bleach is not acceptable for use in pools and hot tubs due to the high level of contaminants found in these products.
Calcium hypochlorite
Calcium hypochlorite is a white, granular powder made by mixing chlorine gas with calcium hydroxide, then drying to the resulting powder.
When added to water, calcium hypochlorite has the following chemical reaction:
Ca(OCl)2 + 2H2O → 2HOCl + Ca++ + 2OH–
Advantages
- compact and easily handled; and
- minimal decomposition in storage.
Disadvantages
- creates strong acid demand by raising pH;
- increases calcium hardness by 4 ppm/lb/37,854 L (10,000 gal); and
- may create cloudy water due to inert insoluble substances or sudden rise of pH causing formation of a CaCO3 ‘cloud.’
Lithium hypochlorite
Lithium hypochlorite is a white, relatively odorless granular solid made by mixing chlorine gas with caustic soda, then with lithium hydroxide, and then drying to a powder.
When added to water, lithium hypochlorite has the following chemical reaction:
Li(OCl) + H2O → HOCl + Li+ + OH–
Advantages
- safest chlorine chemical to handle;
- no premixing required;
- completely soluble; and
- dissolves quickly at normal pool water temperatures and will not bleach vinyl liners.
Disadvantages
- creates strong acid demand by raising pH;
- most costly form of inorganic chlorine; and
- high total dissolved solids (TDS) contribution due to inert content (71 percent).
Trichloroisocyanuric acid (Trichlor)
Trichlor, an organic chlorine, is a slow-dissolving, white solid generally sold in 25 mm (1 in.) and 76.2 mm (3 in.) tablets for automatic erosion or floating feeders.
When added to water, trichlor has the following chemical reaction:
C3N3O3Cl3 + 3H2O → 3HOCl + C3H3N3O3
Advantages
- good for feeders due to slow dissolution; and
- convenient to handle, but use of gloves is recommended.
Disadvantages
- lowers total alkalinity;
- creates strong base demand due to low pH; and
- steadily increases the cyanuric acid (CYA) level, which, if not watched carefully, may result in over-stabilized (chlorine lock): 7-ppm increase/lb/37,854 L (10,000 gal).
Sodium dichloroisocyanurate (Dichlor)
Dichlor is a rapidly dissolving white granular solid generally sold in 25-mm (1-in.) and 76.2-mm (3-in.) tablets for automatic erosion or floating feeders. This type of chlorine is also available in granular form. Its impact on pH is minimal.
When added to water, dichlor has the following chemical reaction:
NaC3N3O3Cl2 + 3H2O → 2HOCl + C3N3O3 + NaOH
Advantages
- easily handled; and
- little impact on acid or base demand due to small pH impact on water.
Disadvantages
- most costly ‘stabilized’ chlorine; and
- will steadily increase the CYA level (rapidly, if used as a shock) which, if not watched carefully, may result in over-stabilized (chlorine lock): 6- to 7-ppm increase/lb/37,854 L (10,000 gal).
Chlorine stabilization
To slow down the natural degradation of unstabilized chlorine by the sun’s ultraviolet (UV) light, cyanuric acid is added to the water (whether supplemented separately when using inorganic chlorine or as part of the physical makeup of organic chlorine in trichlor and dichlor). Cyanuric acid is an odorless, white, granular substance with a maximum solubility of 1600 ppm in water. It is a weak acid when dissolved and will provide protection from UV light for hypochlorous acid in outdoor pools/hot tubs. An ideal concentration of 30 to 50 ppm CYA will permit HOCl to last three to five times longer under sunny conditions than unstabilized water. Cyanuric acid is not appropriate for indoor pools/hot tubs.
Currently, there is no pool chemical available proven to be effective in removing excess CYA. The accepted methods for reducing high levels are to drain and add fresh water that does not contain CYA or use reverse osmosis systems to reduce all total dissolved solids. Splash out, carryout, or backwashing will only lower the levels slightly.
Bromine
Bromine is the other effective sanitizer and oxidizer all rolled into one product. It, too, is available in a variety of forms.
Bromochloro-dimethyl-hydantoin (BCDMH)
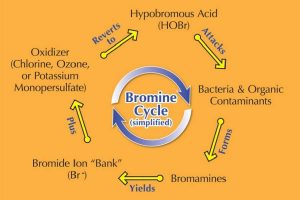
Figure 3: Bromine Cycle
Bromine is most commonly sold as a slow-dissolving tablet used in an erosion feeder or surface floater. Also referred to as ‘organic bromine,’ BCDMH has a pH of 4.5 to 4.8. Notice the letter ‘C’ in the product, indicating BCDMH has a small amount of chlorine as part of its makeup. This is added since bromine needs an oxidizer (chlorine, ozone, potassium monopersulfate) to give it a kick-start. (see Figure 3, Bromine Cycle [simplified])
When added to water, BCDMH has the following chemical reaction:
BCDMH + H2O → HOBr + HOCl + DMH
Dibromo-dimethyl-hydantoin (DBDMH)
This form of bromine is sold as a slow-dissolving nugget or tablet to be used in an erosion feeder or surface floater. With a nearly neutral pH of 6.6, DBDMH has no chlorine content to give it a kick-start; therefore, an oxidizer must be added to an approved feeder or floater.
When added to water, DBDMH has the following chemical reaction:
DBDMH + H2O → HOBr + DMH
The bromine cycle
As mentioned earlier, bromine needs an oxidizer to generate the bromine cycle (see Figure 3, Bromine Cycle [simplified])—whether the oxidizer is part of the product itself (BCDMH) or is added separately (DBDMH).
Advantages
- no need to measure ‘bromamines’ (or combined bromine) as they are good sanitizers in their own right (about 87 percent as effective as HOBr) with an approximate half-life of 20 minutes;
- does not break down in hot water environments as fast as chlorine;
- preferred sanitizer for hot tubs because it is less pH-dependent and does not dissipate quickly in hot water; and
- great for indoor environments because there is no smell or irritations associated when used properly.
Disadvantages
- has no commercially available stabilizer;
- dissipates faster in outdoor environments than stabilized chlorine;
- overdosing bromine may lower total alkalinity; and
- is a weaker oxidizer than chlorine; in hot water environments particularly, shock with chlorine or monopersulfate regularly to prevent rashes.
Other available methods for sanitizing and oxidizing
1. Electrolytic salt chlorine generators (ECGs)
- ECGs pass an electric current through water containing 2500 to 5000+ ppm of sodium chloride (NaCl), forming chlorine gas (Cl2) at one electrode and sodium hydroxide (NaOH) at the other electrode.
- Cl2 reacts with water (H2O) to produce hypochlorous acid (HOCl).
- More than 3700 ppm sodium chloride will give water a slightly salty taste.
2. Ozonators
- Ozonators use either corona discharge or ultraviolet (UV) light to generate ozone (O3). In each process, air runs through a chamber, and oxygen is converted to O3, an unstable molecule.
- O3 is an oxidizer, not a sanitizer. A supplemental sanitizer must be used—generally chlorine or bromine.
- O3 has a half-life of about 18 minutes, so residuals cannot be maintained for normal disinfection.
3. Ionizers
- Ionizers generate copper and silver ions to provide algaecidal and extremely limited biocidal properties. Biocidal effectiveness is solely related to solubility of metal ions.
- An ionizer is not an oxidizer/sanitizer system; it requires a supplemental level of chlorine.
- Do not use bromine with ionizers as silver bromide may form. Silver bromide is potentially hazardous if ingested. It can also damage acrylic/ fiberglass pool and hot tub shells.
4. Polyhexamethylene biguanide (C8H17N5)n
- Polyhexamethylene biguanide (PHMB) is the generic name for the third chemical approved by the Environmental Protection Agency (EPA) as a disinfectant (sanitizer) used for microbiological control.
- Biguanides lack oxidation capability; therefore, to oxidize the organics, hydrogen peroxide (H2O2) is added because of its compatibility with biguanide.
- PHMB is not compatible with chlorine- or bromine-releasing chemicals, copper algaecides, and monopersulfate (MPS).
5. Potassium monopersulfate (KHSO5)
- Potassium monopersulfate is an oxygen-based oxidizer (not a sanitizer) used to remove organic buildup.
- Effectively removes organics that cause chloramine formation.
- Eliminates existing chloramines.
- Will not bleach vinyl liners.
- Dissolves quickly.
- Produces no odors like chlorine products.
- Swimmers can return to the water quickly after application.
Pool and hot tub water chemistry can be a complex subject. This article provides service technicians with an intermediate look at pool and hot tub water chemistry and testing. In a subsequent article, the author will discuss water balance and testing do’s and don’ts.
This article was written by Wayne Ivusich and originally appeared on Pool & Spa Marketing [link].
