Water is an amazing, mysterious, and powerful element. To understand the capabilities of water and its effect on pools it is vital to understand how it travels throughout the world. The water cycle is a continuous loop of the various ways it transforms and renews itself.
Water can exist in three forms: vapor, solid, or liquid. For example, fog is a vaporous form of water as it rises into the atmosphere. As water transforms and travels it is subject to the environment in which it passes. Fog, for instance, rises up from the ground and passes through the surrounding air. During this process, it picks up whatever is in its path (e.g. smoke, dust, or car exhaust). Snow can also pick up contaminants and bacteria and, as it melts into a liquid form, can be transported into nearby rivers and/or streams, or even end up in source water. In fact, in 1993, one of the largest outbreaks of Cryptosporidium in drinking water occurred in Milwaukee and was found to be the result of contaminated snow-melt feeding into Lake Michigan.
Water moves about this planet in many ways and, during this process, it is a great absorber and transporter of whatever it comes in contact with. Today, in this crazy climate, the challenges of source water contamination in pools can be many.
The evaporation problem
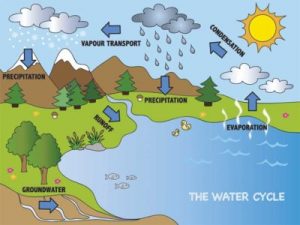
The water cycle is a continuous loop of how it transforms and renews itself.
The average backyard pool contains approximately 68,137 to 75,708 L (18,000 to 20,000 gal) of water. An uncovered pool can lose roughly 6.35 mm (0.25 in.) of water per day—51 mm (2 in.) per week—due to evaporation. In a 68,137 L-pool, this is equal to losing 942.5 L (249 gal) per week and close to 45,425 L (12,000 gal) per year.
When water evaporates from a pool it changes from a liquid state into a gas or water vapor that travels back into the atmosphere. When water vaporizes, only pure water leaves the pool. As this happens, it also leaves behind more dissolved solids, which contribute to total dissolved solids (TDS).
There are two environmental factors which increase the acceleration of evaporation—temperature and humidity. For example, if a pot of water was placed on a table in a cool, humid environment, it could take weeks, even months, before it evaporated. However, if the pot was placed on the stove with the element on, it would evaporate in a matter of minutes. The more heat, the faster evaporation occurs.
Further, when heat is combined with dry air, water will vaporize more quickly. This is why evaporation is a big problem during times of drought when the air is warmer and drier.

When water evaporates from the pool, it changes from a liquid state into a gas that travels back into the atmosphere.
The evaporation rate is higher in southern areas than it is in the north. An evaporation rate study, conducted between 1957 and 1966, found the evaporation rate to be the highest in regions such as southwest Saskatchewan and southeast Alberta where approximately 900 mm (35 in.) of water was lost from lakes and ponds annually. For pools in these regions, increased evaporation means the buildup of solids in the water will also increase, leading to higher TDS, calcium hardness, and pH. As a result, more acid is needed to maintain proper water balance during these drier, hotter days.
Also, as TDS increases, the effectiveness of chlorine (Cl) is reduced. In fact, a TDS that is 1500 parts per million (ppm) over the start-up water can lead to a 50 percent reduction in the effectiveness of chlorine as a sanitizer. Unfortunately, if a region has declared an extreme drought, then draining of pools can be restricted. If the pool cannot be drained and diluted to lower the TDS, 50 percent more chlorine will need to be added to the water just to keep a residual of free available chlorine (FAC).
Proactively reducing organic contaminants through weekly shocking and the use of a natural-based polymer clarifier can help to slow down the buildup of these solids. Also, since phosphates are a part of TDS, using a weekly phosphate removal product can help keep levels lower.
Lower water levels
Another problem during drought periods is groundwater gets used up more quickly. Groundwater is what comes from rain that permeates through the soil and then makes its way below ground through cracks and fissures. As groundwater levels get lower, more contaminants are found in the source water which can lead to higher total hardness, minerals, and metals.
According to the National Groundwater Association (NGWA), almost half of the U.S. population gets its drinking water from groundwater. The concentration of metals, such as copper (Cu) and iron (Fe) increase in deep groundwater. Stain and scale inhibitors, as well as metal sequestering treatments, will need to be used, particularly in areas using well water.
Further, as groundwater levels drop, chloride levels increase from an influx of brackish water. This leads to more particulate and higher dissolved solids. The use of shock treatments and natural polymers to floc and remove these to the filter can help. Nitrates can also be a big problem in groundwater, which can have a direct effect on FAC in pool water. Nitrates create chlorine demand which devours FAC levels. Heavy shocking may in these cases, but draining and using trucked in water, which is free of nitrates, would be the best solution.
The overnight change of make-up water

Due to the impurities in rainwater, chlorine demand can become much greater and sanitizer levels can be reduced to zero.
Today, with water being imported and exported all over the world, it is possible for the make-up of source water to change within hours. In some areas, water is brought in as a supplemental supply from hundreds of kilometers away where the pH, TA, and calcium hardness can differ drastically from the local water source.
Recently, for example, customers in the East Bay area of Northern California noticed a different odor and taste of their tap water. As customers started to complain, the municipality responded by letting customers know that due to the severe drought they needed to bring additional water in from the Sacramento River to supplement their current supply. The officials stated the water brought in had a different make-up from the existing water.
In cases such as this, if the water is not treated properly, it can cause water balance issues in pools. A prime recommendation for pool professionals would be to keep track of the source water by testing from the tap on a regular basis. This will help provide a better understanding of how to treat pool water in a particular area affected by drought.
How fires can affect pool water

Testing and treating pools for phosphates could be paramount immediately following forest fires.
Forest fires are occurring more often in various regions across North America and, thanks to humid, dry weather, are becoming much more devastating.
Recently, Northern California experienced one its worst wildfire seasons ever recorded. In this case, three major fires were raging simultaneously, encompassing more than 100,000 ha.
How do wildfires affect source water, and pools for that matter? Well, the ash from forest fires can be high in nutrients such as nitrates and phosphates, while one of the main firefighting chemicals dropped from planes contains compounds of phosphate. As a result, these elements make their way into the source water in these regions where pool service professionals may see large spikes in phosphate levels in pool water. Therefore, testing and treating pools for phosphates could be paramount immediately following forest fires. Phosphate removers can be very helpful in these situations.
What to expect when the weather goes from dry to wet
Every service professional knows the importance of keeping pool water balanced; however, what might not be known is the importance of adjusting water balance prior to the first rainfall of the season.
Acid rain is a phenomenon many have heard about as a result of pollution in the atmosphere. During dry seasons, there can be a buildup of nitrogen (N) and sulfur (S) compounds in the air. When the moisture from rain or fog combines with these compounds, the result is nitric and sulphuric acid (H2SO4).
When acid rain enters pool water it will have an immediate effect in lowering the pH. This explains why many times after the first heavy rainfall of the season service professionals report a drop in pH in many of the pools they maintain.
To prevent this from happening, service professionals should make sure the TA levels in the pool water are correct before the rain begins (TA is the buffer to resist pH changes). By making sure the pool water’s TA levels are in the 80 to 120 ppm range, it can help prevent the pH from dropping when acid rain hits.
What is in rain?
Despite what some may think, rainwater is not pure. In fact, water is incapable of condensing into droplets without the presence of dust, smoke, or other impurities in the atmosphere. A cloud of water droplets can only form in the presence of impurities. Therefore, any rain that falls into the pool is carrying organic and inorganic substances, bacteria, and nitrogenous compounds of minerals and metals.
Further, after it rains, it is typical for algae to become a problem in pools. This is because rain can increase nitrate levels, which are a known food source for algae. Also, due to the impurities in rainwater, chlorine demand can become much greater and sanitizer levels can be reduced to zero. Therefore, after a heavy downpour, it is important to test for nitrates, super-chlorinate the water, and use an all-purpose algaecide.
| Water maintenance guidelines for pools that are under the weather |
|---|
| • During a drought, the saturation of water increases due to low groundwater levels and higher rates of evaporation. • Pool water draining restrictions during times of drought can lead to an increased use of chemicals to maintain sanitary water conditions. • Evaporation is one of the leading causes of pool water loss when it is warm and dry. • Solid or liquid covers pool can help reduce evaporation. • Water sources can change rapidly during times of drought; therefore source water should be tested regularly. • The first rainfall of the season can introduce a number of contaminants to the pool which will have an effect on the water chemistry. • The acid content of rain can be higher during the first rainfall and can have a direct affect on pH. • Manage alkalinity levels prior to heavy storms. • Flood waters can introduce various contaminants to the pool, including raw sewage that can contain bacteria and Cryptosporidium. • Phosphate levels can increase after a heavy wildfire season due to ash and the use of fire retardant chemicals. • Proactive shocking, phosphate removal, clarifier, and algaecide use can help in all-weather scenarios. |
The hidden dangers of flood water

Whenever floodwaters enter a pool, it is best to drain all of the water.
Several types of flooding can occur. Flash floods are the result of a break in a levee or dam or heavy rains in mountain areas above a floodplain. Flooding can be very destructive and cause contaminated water to rush quickly and overwhelm low-lying areas.
In coastal regions, storm surges and high tides can also cause flooding. For example, when Hurricane Sandy hit the coast of New Jersey, many service professionals reported finding live shrimp in their client’s pools after the floodwaters receded.
Floodwaters contain mud, silt, organic materials and, quite possibly, raw sewage that is loaded with bacteria. Whenever floodwaters enter a pool, it is best to drain all of the water. If this is not possible, the pool should be super-chlorinated to at least 20 ppm of chlorine at a pH of 7.5. The pool should be kept at 20 ppm for at least 15 hours to ensure all pathogenic organisms have been inactivated.
The use of a natural polymer flocculent can also help clear materials out via the pool filter. The filter should be thoroughly cleaned once the pool water has cleared. In agricultural areas or in phosphate mining regions, service professionals may also expect to find mass levels of nitrates and phosphates from floodwaters.
Be ready
There is no denying weather patterns are changing and in certain areas water itself has become a big challenge. Groundwater levels have sunk to catastrophic levels and regulators have imposed strict conservation laws as a result. The building, filling, or draining of pools has come under extreme scrutiny from many officials in these regions. Despite the fact it has been proven that pools require less water to maintain than a backyard lawn, they still fall in the crosshairs of lawmakers and private citizens.
Pool professionals need to be diligent during these times to ensure the industry does not become falsely targeted as water wasters.
Having a plan in place on how to conserve and prevent waste of pool water is needed ahead of time. As mentioned earlier, one of the largest water wasters is evaporation; therefore, if service professionals are diligent in doing what they can to reduce the evaporative loss of pool water, it goes a long way in demonstrating the industry’s pro-conservation efforts.
Solid covers work best; however, if they are not an option, a chemical liquid cover can be applied to help prevent water loss.
When the rains of El Nino start to move in, it is important to be prepared by making sure all pools are properly balanced. Super chlorinate after periods of heavy rains or flooding; test pool water for nitrates and phosphates, and be sure to use a good quality algaecide after the rains have passed.
The weather is hard to predict, but if service professionals are ready for anything, it can help them prevent pool water problems before they begin.
This article was written by Terry Arko and originally appeared on Pool & Spa Marketing [link].
