Despite some long-held beliefs, a hot tub is not simply a small pool. There are major differences between the chemistry of these aquatic environments that are unique enough to warrant a separate discussion. For service technicians, understanding these differences and knowing how to alter a testing and treatment regimen geared for pools to one suited for hot tubs is critical for maintaining healthy water for their customers.
How does hot tub water differ from pool water?
Although pools and hot tubs are both filled with water and require regular testing and treatment, this is where the similarities end. Higher temperature, smaller volume, jetted water, and the likelihood of high/dense bather loads, must all be taken into account when formulating an appropriate testing and treatment regimen for a hot tub. When it comes to water chemistry, however, the two most significant differences are higher temperatures and smaller volumes.
Higher temperatures
The temperature currently recommended by the industry for pool water is 25.5 to 27.8 C (78 to 82 F). For spas, the recommendation is a temperature no higher than 40 C (104 F). Temperatures greater than 40 C (104 F) can lead to major health issues, such as hyperthermia.
Higher temperatures also have a tendency to increase bacterial growth, such as Pseudomonas aeruginosa, the most common bacteria found in hot water environments. In warmer water, skin pores open, allowing these bacteria to enter the body. As a result, mild skin rashes (sometimes referred to as ‘hot tub rash’) and ear infections can occur when the sanitizer is not maintained at the proper level.
Keep in mind, for every 10-degree increase in water temperature, chemical reactions will increase twofold, which explains why sanitizers are said to be short-lived in hot tub water due to the quicker depletion rate.
Another factor to consider is the greater tendency for calcium hardness to come out of solution, resulting in crusty deposits on surfaces and equipment, clogged filters, reduced circulation, and cloudy water. For those reasons, the ideal calcium hardness level recommended for hot tubs is only 150 to 250 parts per million (ppm), whereas the recommended level for pools is 200 to 400 ppm.
Approximately 50 per cent of water loss in pools and hot tubs is due to evaporation, and the jetted water in hot tubs increases that rate. As a result, the decreased water volume generates an increase in the accumulation of total dissolved solids (TDS). Although a minor component of overall water balance, TDS can present a problem if allowed to buildup: It can slow down chemical reactions, contribute to the corrosion of pool components, and create dull, uninviting water.
Further, natural body oils, perspiration, etc., are expelled at a faster rate in warm water than in the cooler temperatures found in a pool. Heated water encourages more urine/sweat production—an average of 50 mL (1.7 oz) of urine and 200 mL (6.7 oz) of sweat during an average 15 to 20 minute soak. Therefore, the importance of maintaining proper sanitizer levels cannot be emphasized enough.
Smaller volumes
‘Teaspoon chemistry’ is a term some service technicians use to describe how hot tubs should be chemically treated. Due to the smaller water volumes, lesser amounts of treatment products are added as opposed to a pool—teaspoons of this or capfuls of that—as opposed to kilograms of this or litres of that. Many hot tub owners think that more is always better, so they add more treatment product(s) than necessary, which only creates more problems.
Hot tub volumes vary from as low as 567 L (150 gal) for residential hot tubs to as high as 9464 to 11,356 L (2500 to 3000 gal) or more for commercial units. No matter the volume, the turnover rate (the amount of water in gallons/litres that flows through the entire system once) is anywhere from 15 to 45 minutes for hot tubs compared to six to eight hours for pools.
Aeration (jetted water) also causes pH to rise by introducing additional hydroxide ions (OH–) into the water. Careful and consistent pH testing is needed to make sure the increased pH level does not affect water balance or cause cloudiness/scaling.
Water balance in hot tubs
The five parameters of water balance are pH, total alkalinity (TA), calcium hardness, temperature, and TDS. These factors are then used in the Langelier Saturation Index (LSI or SI) to obtain a number which represents whether the water in a hot tub has a tendency to be corrosive or scale-forming.
Langelier Saturation Index
SI = pH + TF + logCH + logALK – Constant
- SI = Saturation Index
- pH = measured pH
- TF = temperature factor
- CH = measured calcium hardness
- ALK = measured carbonate alkalinity
- CONSTANT = combined factor for temperature and ionic strength correction, and concentration conversions.
Ideal ranges recommended for hot tubs
- Free chlorine = 3 to 5 ppm
- Combined chlorine = 0 ppm
- Bromine (Br) 4 to 6 ppm
- pH 7.4 to 7.6
- TA 80 to 120 ppm
- Calcium hardness 150 to 250 ppm
- Maximum temperature 40 C (104 F)
- TDS no greater than 1500 ppm above source water TDS level
Hot tub water is considered ‘balanced’ if the result of the SI calculation falls between -0.3 and +0.5. Positive values greater than +0.5 can indicate the potential for cloudy/scaling water. Negative values less than -0.3 can indicate the potential for corrosive water.
If hot tub water (or pool water for that matter) has an SI lower than -0.3, it will naturally attempt to balance itself by seeking calcium carbonate (CaCO3) from any source in its quest (concrete, plaster, and especially grout or gunite), leaving behind pits, holes, and other signs of deterioration. Although this is not as much an issue since most hot tubs have fibreglass/acrylic shells, the water will still behave aggressively and attack any metals in the system, such as heater tubes, causing corrosion and eventual failure of the metals.
The ultimate solution for any hot tub water problem
Despite well-meaning attempts to properly sanitize, oxidize, balance, and filter hot tub water, at some point (because of the combination of smaller volumes and larger bather loads) the hot tub will need to be drained and refilled with fresh water.
In the ’90s, a formula was developed to determine how often hot tubs should be drained and refilled with fresh water. This formula is a guideline for draining residential and commercial/public hot tubs. The first step is to convert litres to gallons by multiplying by 0.26. Next, take one-third of the total gallons of water in the hot tub and divide it by the maximum number of daily bathers (‘bather’ equals one 20-minute soak) to get the number of days between complete draining/refilling:
# of days = 1/3 volume (gallons) maximum # daily bathers
Scenario #1
- A residential customer has a 2,271-litre hot tub (= 600 gal).
- 600 ÷ 3 = 200
- Every day, two people use this hot tub (200 ÷ 2 = 100).
- Therefore, the hot tub should be drained and refilled every 100 days.
Scenario #2
- A commercial customer (e.g. health club) has a 5678-litre hot tub (= 1500 gal).
- 1500 ÷ 3 = 500
- Every day, 100 patrons use this hot tub (500 ÷ 100 = 5).
- Therefore, the hot tub should be drained and refilled every five days.
Spa water sanitation/oxidation
When selecting which sanitizing/oxidizing method to use for a hot water environment, there are a few basics that should be understood to provide the customer with the best value.
- Hot water environments deplete chlorine (stabilized or unstabilized) faster than bromine.
- Chemical reactions occur faster in hot water environments than in cooler water environments.
- Treatment chemicals are added in hot tubs in significantly smaller amounts than in pools.
- The ultimate solution to any pool/hot tub chemistry problem is fresh water.
- Organic loads are more concentrated in smaller volume hot tubs; each bather contributes anywhere between 10 and 100 million bacteria to the water.
With the basics out of the way, the most popular options available for sanitizing/oxidizing hot tubs can be discussed.
Chlorine
Chlorine is clearly the preferred sanitizer/oxidizer product in the pool/hot tub industry, as it is readily available in a variety of forms (e.g. powder, granules, liquid, tablets, pucks, etc.). The primary question to ask before using chlorine is whether the hot tub is indoors or outdoors. If indoors, then chlorine is not always the best choice since it can create a multitude of problems if the hot tub is not properly maintained. The poor air circulation combined with a buildup of combined chlorine can cause irritation to the eyes and mucous membranes and also create that smelly ‘chlorine odour’ which is often associated with indoor hot tubs.
If the hot tub is located outside and the customer prefers to use chlorine, remember to use a ‘stabilized’ form (e.g. dichlor or trichlor), which will slow down the decomposition of the chorine due to sunlight.
Dichlor is almost pH neutral at 6.6, while Trichlor carries an acidic pH (2.8 to 3.5). The unstabilized forms of chlorine (e.g. calcium, lithium, and sodium) all have higher pH levels ranging from 8.5 to 13. Understanding the pH levels of products will help to better control the water’s pH levels.
Bromine
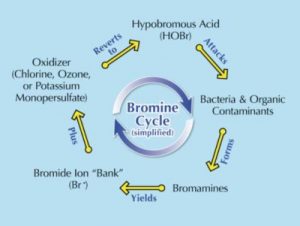
Figure 1: The bromine cycle.
There are four key advantages of using bromine over chlorine in hot tub environments:
- It is more effective over a wider pH range, whereas chlorine’s effectiveness decreases as pH rises.
- Bromamines are almost as effective at sanitizing/oxidizing as free bromine.
- Bromamines, formed in reaction with ammonia wastes, do not have the unpleasant odour of chloramines and are less irritating to the eyes and respiratory tract.
- Bromine lasts longer in hot water environments than unstabilized chlorine.
Generally, bromine can be purchased in tablet form: BCDMH (1-Bromo-3-chloro-5,5-dimethylhydantoin) or DBDMH (1,3-Dibromo-5,5-dimethylhydantoin). Tablets are placed in an erosion feeder (or floater), and as hot tub water spills over the tablets, bromine is introduced into the water. Bromine is unique in that it needs a ‘kick start’ to get it going (like starting a motorcycle or dirt bike). This can be in the form of any oxidizing substance (e.g. chlorine, ozone, or monopersulfate). This in turn starts what is called the ‘bromine cycle’ (see Figure 1).
If BCDMH tablets are used there is no need to add a separate oxidizer—it is already there (the ‘C’ in BCDMH stands for chlorine). If using DBDMH, an oxidizer will need to be added separately.
The one issue with using bromine is that it is slightly acidic. BCDMH has a pH of 4.5 to 4.8 and DMDBH has a pH of 6.6. These lower pH levels can be compensated by following proper water balance practices—specifically, making sure the TA level is sufficiently high (but still within acceptable ranges) to offset the lower pH. Hot tub equipment can be damaged if exposed to low pH levels.
Another important factor to consider is bromine has no commercially available stabilizer; therefore, it is best used for indoor hot tubs or units installed outdoors with a cover.
Ozone (O3)
Ozone is another option for sanitizing hot tubs. Gaining in popularity, many hot tub manufacturers now include ozonators in their units. Ozone is an excellent oxidizer, but its usefulness as a sanitizer is limited as it readily converts to oxygen as soon as it hits the water’s surface. If using ozone, manufacturers recommend using a minute amount of chlorine or bromine to create a sanitizing residual in the water—usually half of what would be used if no ozone were present.
Other sanitizing/oxidizing methods
Ionization is a method that generates copper (Cu) and silver (Ag) ions to provide algaecidal properties and extremely limited biocidal properties. It is important to remember ionizers (a.k.a. mineralizers) are not oxidizer/sanitizer systems. Despite marketing claims, ionizers require a small amount of chlorine to provide a sanitizing/oxidizing residual in hot tub water. Caveat: Do not use bromine with ionizers as silver bromide may form. This chemical compound is potentially hazardous if ingested and can stain acrylic/fibreglass shells.
Polyhexamethylene biguanide (PHMB) products are used primarily for microbiological control. Because PHMB lacks oxidation capabilities, hydrogen peroxide (H2O2) needs to be added as an oxidizer/shock to eliminate any organic buildup. It is important to note PHMB is not compatible with most halogen-based products (e.g. chlorine, bromine, ozone, monopersulfate, and copper-based algaecides).
Potassium monopersulfate (KHSO5) is an oxygen-based oxidizer (not a sanitizer) used to remove organic buildup, prevent new combined chlorine formation, and kick start the bromine cycle. This product dissolves quickly, produces no odours or irritation, and bathers can get back in the spa within 45 minutes to an hour after it is added. Caution should be used with monopersulfate as it has an extremely low pH (2.3), and overdosing can lead to equipment corrosion and/or water balance problems.
Knowing these hot water chemistry basics will help to ensure the water in a customer’s hot tub is in pristine condition. A happy customer can be a company’s best source of advertisement.
This article was written by Wayne Ivusich and originally appeared on Pool & Spa Marketing [link].
